FDA Warns Against Use of Neck Floats After Death of Baby
Baby neck floats are under fire again after the Food and Drug Administration (FDA) warned against their use in water therapies following the death of one baby and hospitalization of another in unsupervised usage.“Do not use baby neck floats for water therapy intervention,” the FDA wrote in a media statement released on June 28. “Especially with babies who have developmental delays or special needs, such as spina bifida, spinal muscular atrophy (SMA) type 1, Down syndrome, or cerebral palsy. The use of these products can lead to death or serious injury.” Though these events are rare, the FDA advised that they can happen, with both of the cases happening when a caregiver was not directly monitoring the baby. Neck floats are inflatable plastic rings that can be placed around babies’ necks and allow them to float freely in the water. Parents and caregivers use these products while bathing a baby or when babies are swimming, and also during physical therapy for babies with developmental delays or disabilities. Some floats are marketed for babies as young as 2 weeks old, as well as for premature babies. The agency said companies are marketing their neck floats for water therapy, claiming improvement in motion, muscle tone, lung capacity, and better sleep, among many other benefits. However, these products have not received official approval or clearance as physical therapy tools, nor has their effectiveness or safety been fully established. Not the First Warning It is not the first-time baby neck floats are under fire from public health scrutiny. In 2017, health experts warned against the use of baby neck floats. Associate Professor Kyran Quinlan of Rush University Medical Center and former chair of the American Academy of Pediatrics (AAP) Council on Injury, Violence and Poison Prevention, called them “potential death traps.” “Neck floats for babies scare me to death, and I hope they scare parents,” he said on WDSU. “These are potential death traps … to have your precious baby one poorly sealed seam away from going under at the pool is frightening.” Other experts also warned against air-filled swimming aids, including armbands, as any deflation takes away their buoyancy. The media scrutinized Otteroo, a major baby neck float company for recalling 3,000 neck floats in 2015 due to 54 reports of deflation. Though the FDA warned that the benefits of a baby neck float as a form of water therapy have not been established, some studies have suggested the use of the company’s neck floats improved motor skills in babies and have potential as a form of aquatic therapy. One study’s author later became a consultant for Otteroo after publishing the paper. The Otteroo website also posts anecdotal testimony from parents who report seeing physical improvements in their premature and developmentally impaired babies’ development since using Otteroo as water therapy. In response to the negative media attention on Otteroo and other baby neck floats in 2017, the company released a statement in 2018 stating that the product “is not a death trap and not dangerous,” but also that the product is not a babysitter, nor a life-saving device, and that it is not for swimming and did not give neck strain. The Epoch Times contacted Otteroo for a comment.
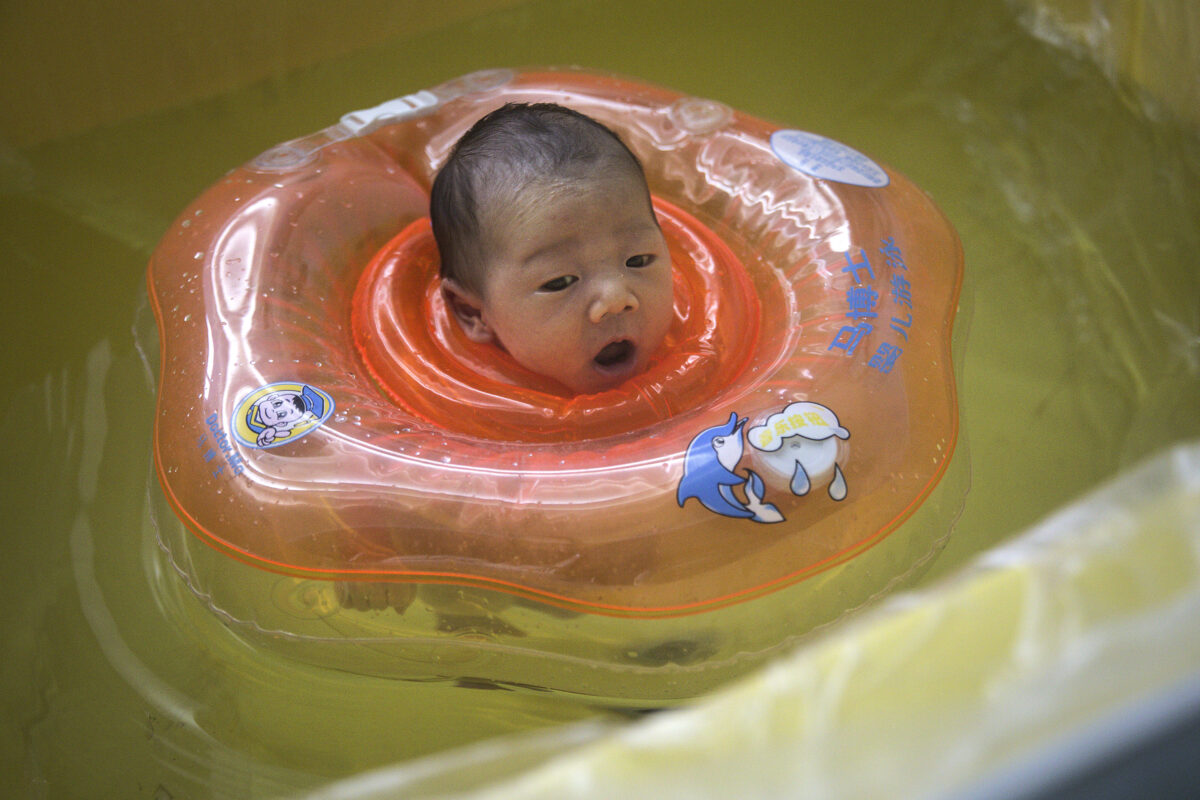
Baby neck floats are under fire again after the Food and Drug Administration (FDA) warned against their use in water therapies following the death of one baby and hospitalization of another in unsupervised usage.
“Do not use baby neck floats for water therapy intervention,” the FDA wrote in a media statement released on June 28.
“Especially with babies who have developmental delays or special needs, such as spina bifida, spinal muscular atrophy (SMA) type 1, Down syndrome, or cerebral palsy. The use of these products can lead to death or serious injury.”
Though these events are rare, the FDA advised that they can happen, with both of the cases happening when a caregiver was not directly monitoring the baby.
Neck floats are inflatable plastic rings that can be placed around babies’ necks and allow them to float freely in the water.
Parents and caregivers use these products while bathing a baby or when babies are swimming, and also during physical therapy for babies with developmental delays or disabilities. Some floats are marketed for babies as young as 2 weeks old, as well as for premature babies.
The agency said companies are marketing their neck floats for water therapy, claiming improvement in motion, muscle tone, lung capacity, and better sleep, among many other benefits.
However, these products have not received official approval or clearance as physical therapy tools, nor has their effectiveness or safety been fully established.
Not the First Warning
It is not the first-time baby neck floats are under fire from public health scrutiny.
In 2017, health experts warned against the use of baby neck floats.
Associate Professor Kyran Quinlan of Rush University Medical Center and former chair of the American Academy of Pediatrics (AAP) Council on Injury, Violence and Poison Prevention, called them “potential death traps.”
“Neck floats for babies scare me to death, and I hope they scare parents,” he said on WDSU.
“These are potential death traps … to have your precious baby one poorly sealed seam away from going under at the pool is frightening.”
Other experts also warned against air-filled swimming aids, including armbands, as any deflation takes away their buoyancy. The media scrutinized Otteroo, a major baby neck float company for recalling 3,000 neck floats in 2015 due to 54 reports of deflation.
Though the FDA warned that the benefits of a baby neck float as a form of water therapy have not been established, some studies have suggested the use of the company’s neck floats improved motor skills in babies and have potential as a form of aquatic therapy. One study’s author later became a consultant for Otteroo after publishing the paper.
The Otteroo website also posts anecdotal testimony from parents who report seeing physical improvements in their premature and developmentally impaired babies’ development since using Otteroo as water therapy.
In response to the negative media attention on Otteroo and other baby neck floats in 2017, the company released a statement in 2018 stating that the product “is not a death trap and not dangerous,” but also that the product is not a babysitter, nor a life-saving device, and that it is not for swimming and did not give neck strain.
The Epoch Times contacted Otteroo for a comment.